Our organs are interconnected through vascular and neuronal networks. We aim to understand how these networks contribute to organ growth, injury perception, and recovery.
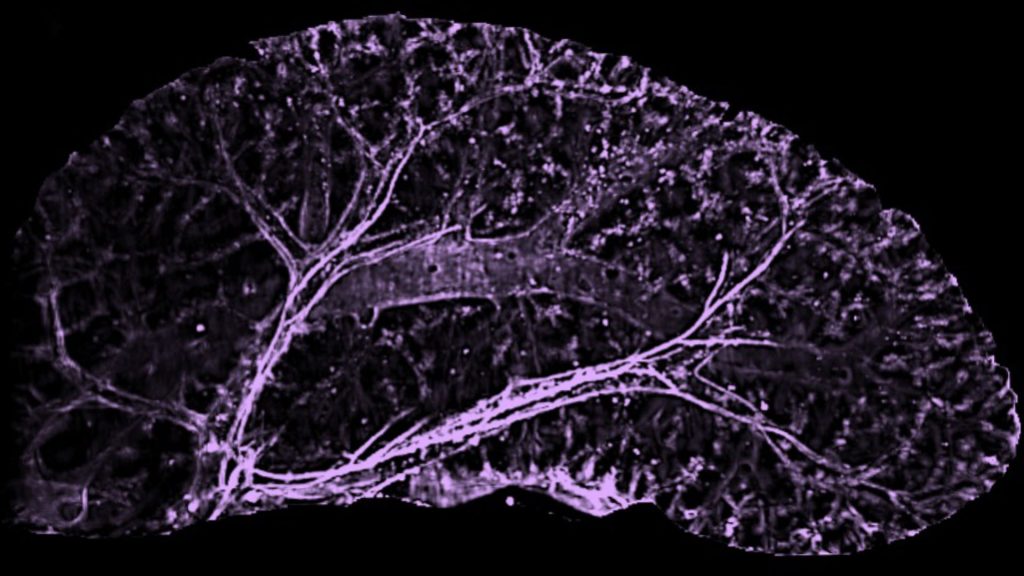
We study this question primarily in the mouse liver. Liver size is set at a stereotyped ratio to body size. When a mouse liver is transplanted into a rat, for instance, the organ grows until it matches the size of a rat liver. Such remarkable growth capacity of the liver offers a unique opportunity to study the mechanisms of organ growth and size control. How do the liver cells know how much to grow?
We have found that the vasculature plays a key role in determining liver size during postnatal growth. The liver is a collection of highly vascularized units termed lobules. We identified a population of vascular progenitors present in the mouse liver only for a short period after birth. These progenitors vascularize newly forming lobules. Once the vascular progenitors are lost, no more new lobules are added to the organ, setting final liver size.
We are now expanding on this work to identify the molecular signals behind liver vascularization and size control during normal growth, and the role these mechanisms play during liver regeneration.

Current Projects
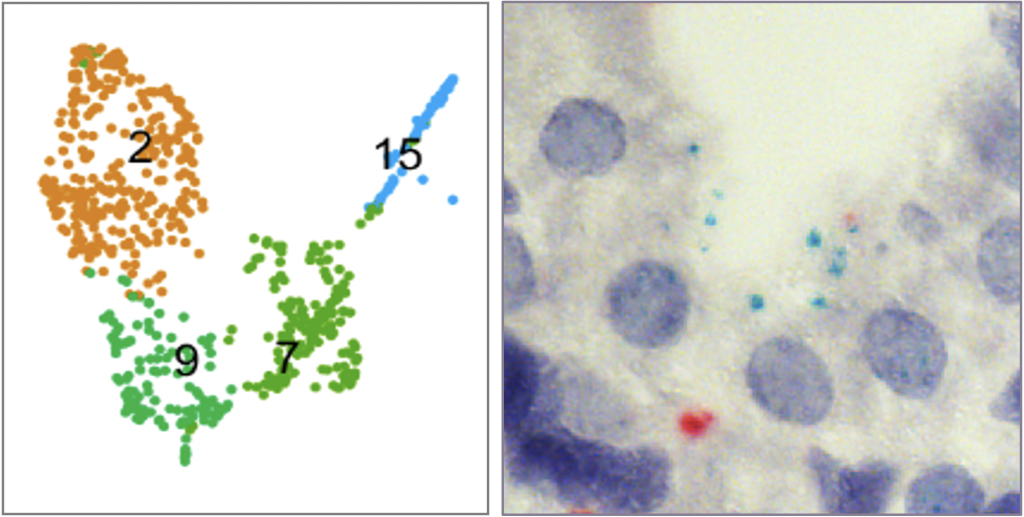
One of the avenues in our lab focuses on understanding the molecular mechanisms behind rapid growth and size control of the postnatal liver. The vascular progenitors we have identified are the main source of liver vascularization after birth. These progenitors decline sharply well before adulthood, setting a limit on vascularization. This cellular mechanism pre-determines the final size of the organ. How are the vascular progenitors lost? We have performed single cell RNA-sequencing to dissect the key signals behind progenitor decline. We are now studying these signaling mechanisms.
We complement these studies with in vitro organoid work that focuses on generating true liver organoids. Our goal is to re-create a full lobule in vitro by seeding the vascular progenitors we have identified along with hepatocytes and other cell types.
These studies will improve our understanding of the principles that govern organ growth and size control. What we learn from here will serve two critical goals of translational research; generating vascularized organoids, and mimicking liver function for drug research.
Vascularization in Organ Regeneration
The liver has remarkable capacity to recover following partial removal or intoxication. How does the organ bring back its blood vessels and coordinate their re-growth with other cells to make functional tissue? We are currently studying the role of different vascular subtypes and progenitor cells in vascularization and recovery of the liver following chemical or physical injuries.
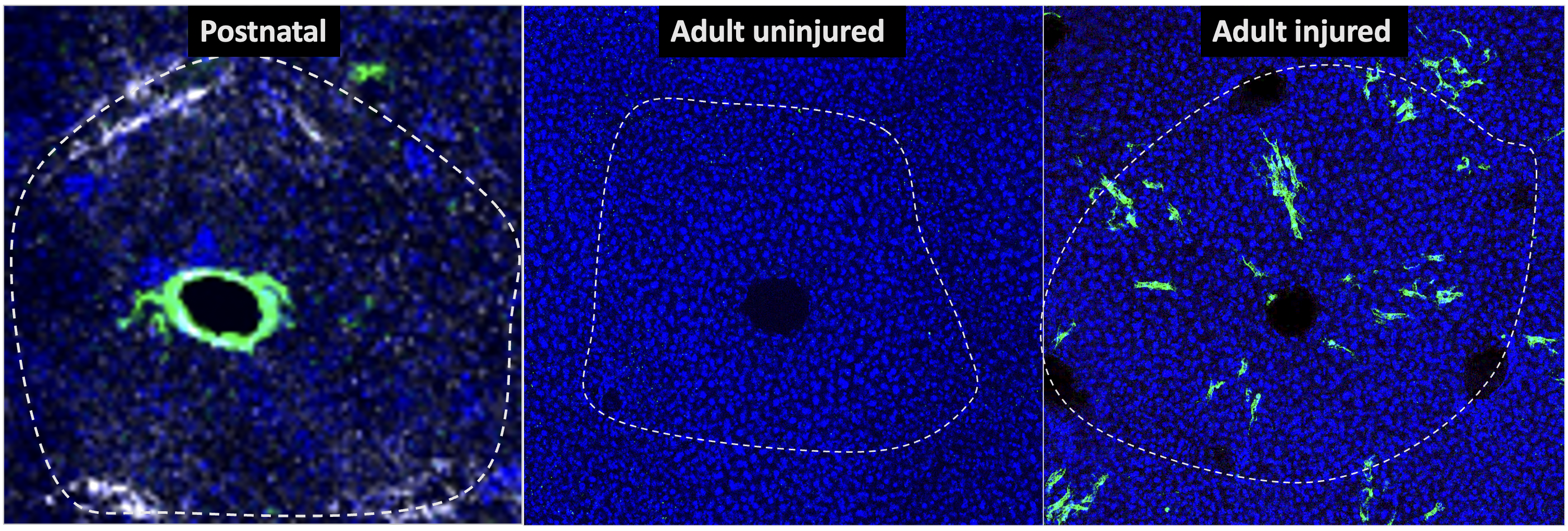
Innervation in Organ Regeneration
An exciting new avenue in the lab explores how liver innervation impacts response and regeneration following injury. We have found that the liver is highly innervated alongside blood vessels. Our observations point at close communication between liver innervation and the vasculature, particularly following injury. We are currently working to understand what signals are communicated between nerves and blood vessels of the liver, and how these signals impact injury response of the organ. These studies are revealing new cellular players and mechanisms in mammalian organ regeneration.